A straightforward plan for teams that want real progress, not another “AI experiment.”
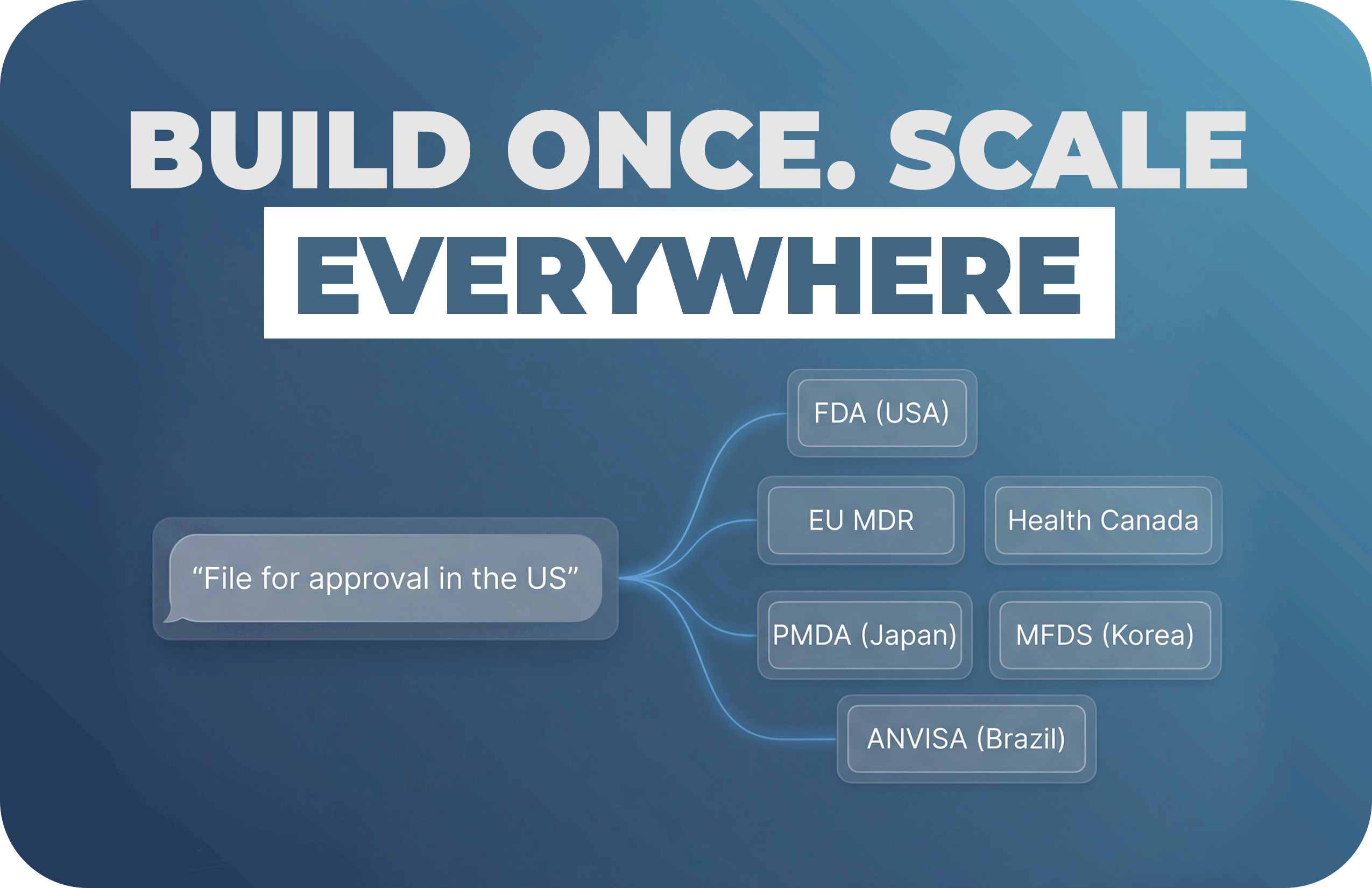

A straightforward plan for teams that want real progress, not another “AI experiment.”

Mastering Regulatory Compliance: Insights from Singapore’s Good Submission Practice Workshop 2024 Si...
-1.png)
Exploring the complex landscape of EU regulations for medical devices and in-vitro diagnostic device...

Malaysia's Medical Device Authority (MDA) has just released an important regulatory update that coul...

On March 18, 2024, the Center for Medical Device Evaluation (CMDE) released an important Notice that...

Brazil's Draft Resolution 1200, dated September 1st, 2023, outlining a streamlined process for evalu...

In a move aimed at bolstering the quality management of medical device operations across China, the ...

On November 20, 2023, a significant development unfolded in the realm of medical device regulation a...

Announcement on the Medical Device Administrative Management System Accepting Sales Approval from th...

On February 5, 2024, the Saudi Food & Drug Authority (SFDA) marked a significant milestone with the ...