A straightforward plan for teams that want real progress, not another “AI experiment.”

A straightforward plan for teams that want real progress, not another “AI experiment.”

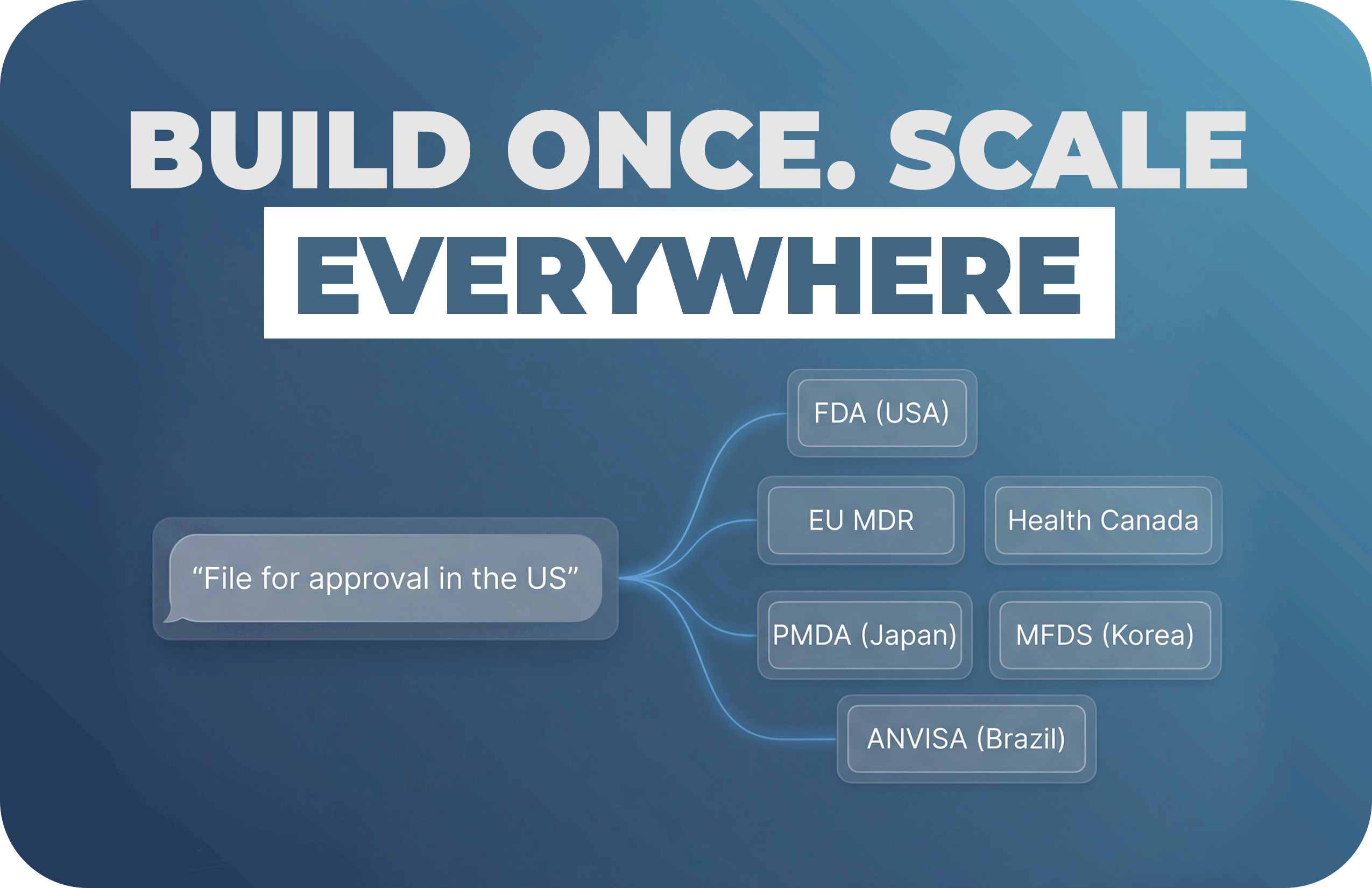
How a Small RA Team Should Measure AI Impact, Choose the Right Level of Adoption, and Navigate Trans...



Case Study: Regulatory Affairs in MedTech

Strengthening Compliance: Taiwan Releases Preclinical Testing Standards for Key Medical Devices
.png)
Here are essential deadlines, new post-market surveillance rules, and compliance strategies for unin...

Accelerating Innovation: Saudi Arabia’s Fast Track Pathways for Innovative Medical Devices


Mastering Regulatory Compliance: Insights from Singapore’s Good Submission Practice Workshop 2024 Si...