A straightforward plan for teams that want real progress, not another “AI experiment.”

A straightforward plan for teams that want real progress, not another “AI experiment.”

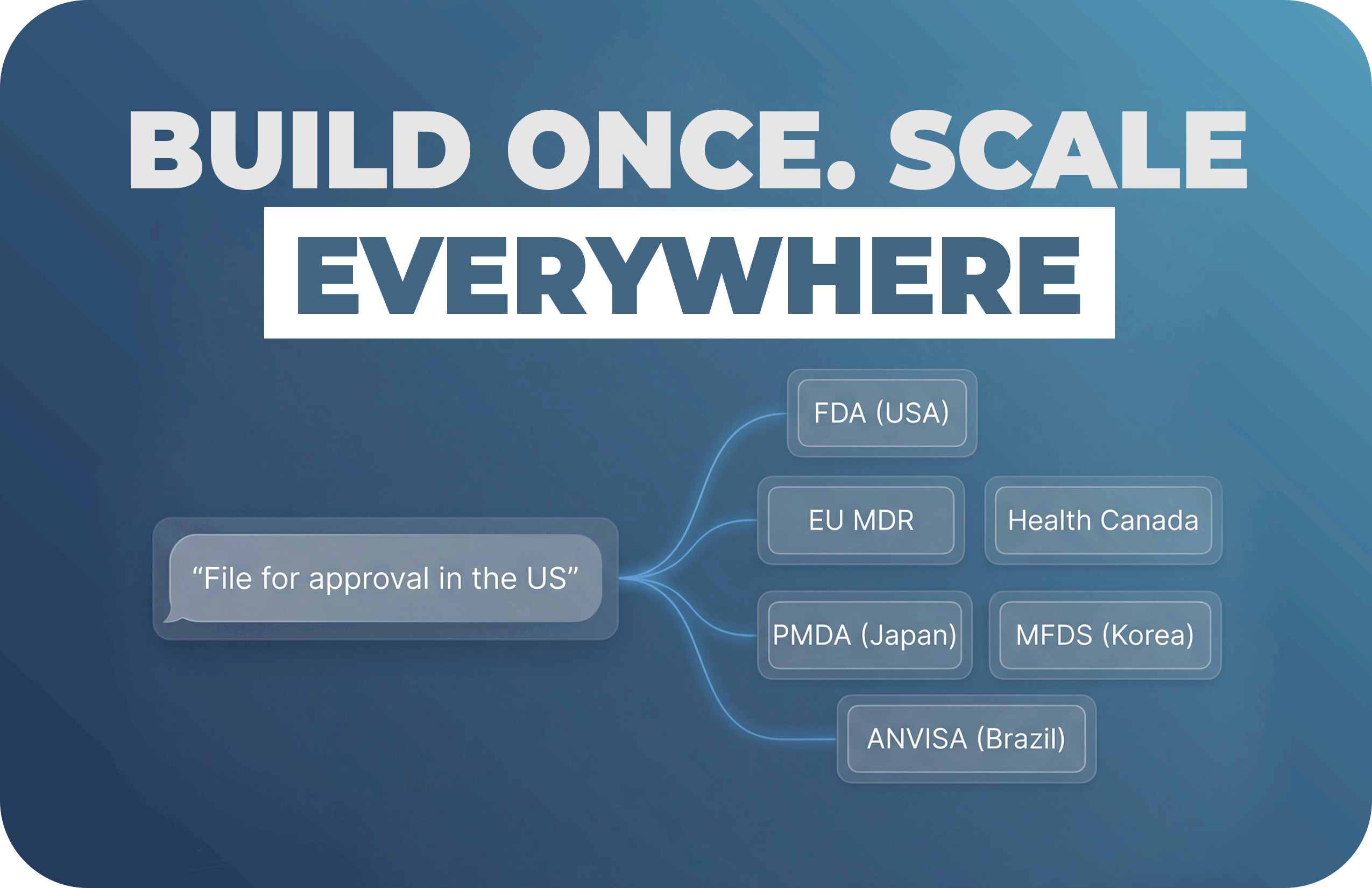
How a Small RA Team Should Measure AI Impact, Choose the Right Level of Adoption, and Navigate Trans...

…. And what they mean for your 2026 budget



Case Study: Regulatory Affairs in MedTech
Artificial Intelligence (AI) has quickly become one of the hottest topics in MedTech. Today’s conver...

Strengthening Compliance: Taiwan Releases Preclinical Testing Standards for Key Medical Devices
.png)
Here are essential deadlines, new post-market surveillance rules, and compliance strategies for unin...

Accelerating Innovation: Saudi Arabia’s Fast Track Pathways for Innovative Medical Devices